Foreseeing the Future: Driving Healthcare Innovation with Edge AI
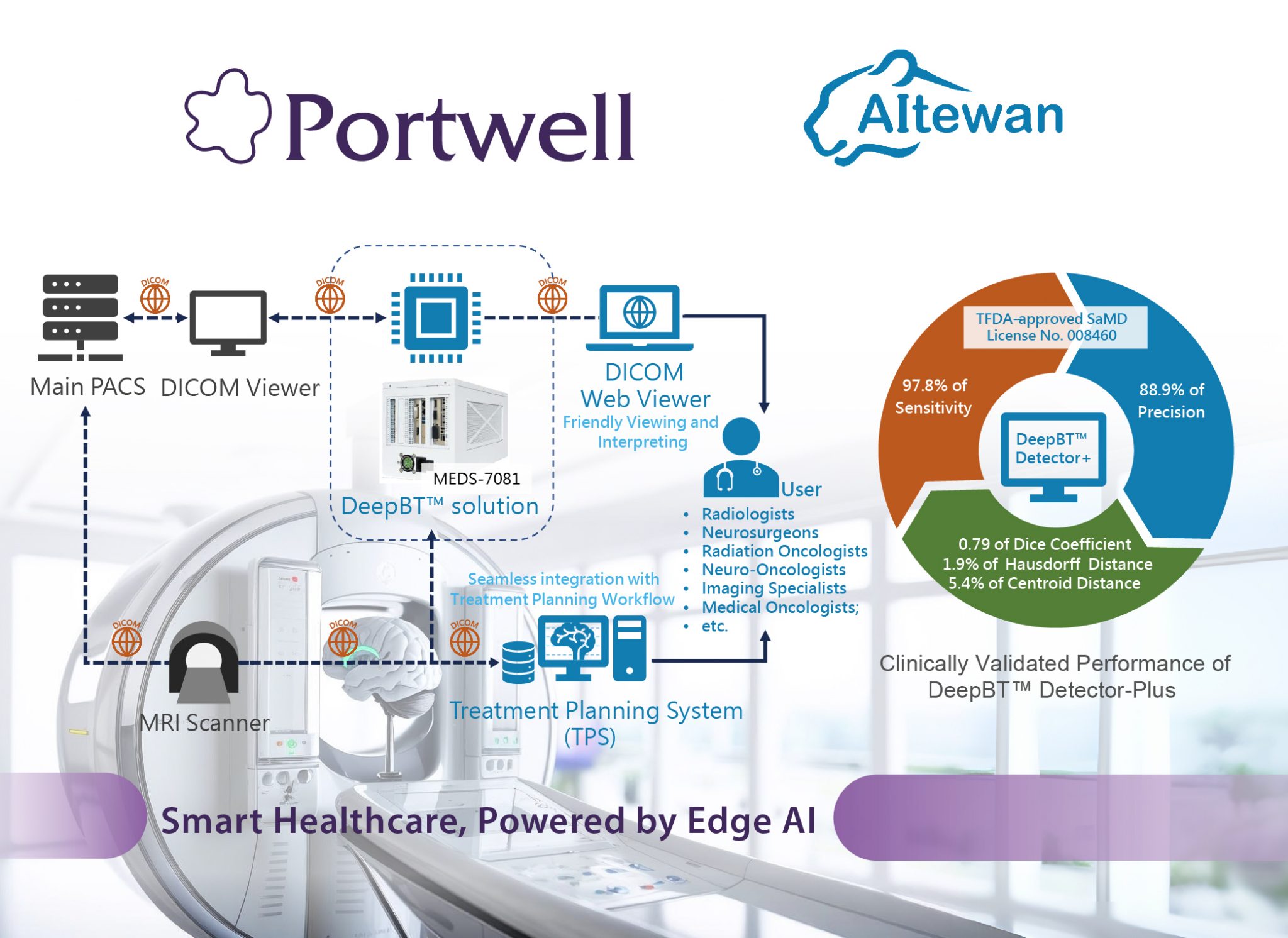
The healthcare industry is shifting toward smart, integrated ecosystems that combine hardware, software, and services. With the rise of AI and edge computing, technologies like CT and MRI imaging analytics now offer efficient, accurate results. In life support, edge AI enables timely risk prediction, improving patient care and reducing clinician