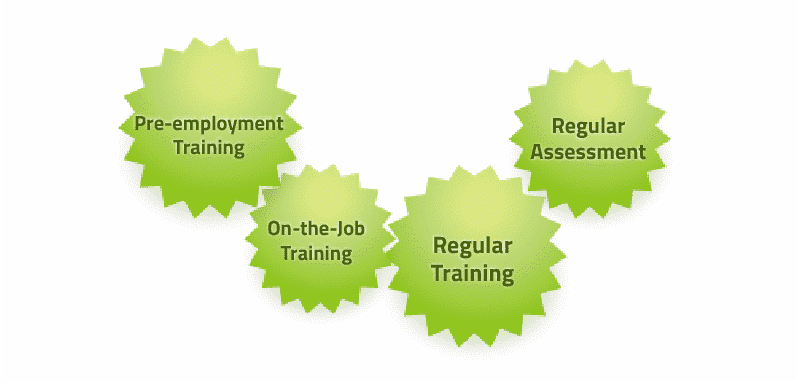
In Portwell MOC, each operator’s professional skills are improved by training before jobs and re-training periodically as necessary. By paying particular attention to the human aspects of production, MOC ensures stable and reliable quality which directly decreases the costs of poor quality and increase customer satisfaction.
Individual training needs to be established based upon job requirements, and re-established whenever new equipment, processes or products are introduced. Training ensures that employees understand the consequences of performing their jobs incorrectly, and is conducted prior to assigning employees, contractors, or temporary personnel to a new task. Training records are maintained according to the quality system.
In the meantime, competency is measured relative to quality trends and retraining is provided where necessary.
As an integral part of the overall quality system, Portwell emphasizes quality during the entire manufacturing process, from the acquisition of material to the delivery of finished goods.
In practice, documents are attached with materials from the Receiving department to IQC inspection or to IQC return if rejected. The internal audit checklist ensures that requirements are met for each process. In addition, Portwell periodically maintains and calibrates equipment. Per the standard process, if any equipment is found out of specification, the last three lots of products will be re-tested using confirmed calibrated equipment. Most importantly for quality control, all procedures include a checklist for inspection within incoming, in-process, and final out-going QC to ensure that correct documents and revisions are in place before assembly. MES software is used to ensure that each assembly station and process step is completed before moving to next step.

In order to ensure Portwell offers world-class manufacturing services, the corrective and preventive processes are implemented to manage abnormalities and potential problems.
The QA member in charge of a quality issue involves the supplier to provide corrective actions upon discovering issues. A Supplier Corrective Action form is sent to the supplier to document the root cause, corrective action and Portwell’s approval. Once the supplier’s corrective actions are returned and approved by the QA team, the document is signed which closes the request in Portwell’s quality system.
Portwell reviews open issues monthly to track issues in order to resolve and provide closure. We provide a complete check on all of unresolved issues and establish a time line to close them.
Portwell ensures customer care by identifying and communicating abnormalities. It is for this reason that corrective and preventive action is taken – to find out the root cause and continuously monitor the effectiveness of the quality system after solutions are implemented to ensure issues do not recur.

To provide the best experiences, we and our partners use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us and our partners to process personal data such as browsing behavior or unique IDs on this site and show (non-) personalized ads. Not consenting or withdrawing consent, may adversely affect certain features and functions.
Click below to consent to the above or make granular choices. Your choices will be applied to this site only. You can change your settings at any time, including withdrawing your consent, by using the toggles on the Cookie Policy, or by clicking on the manage consent button at the bottom of the screen.